Highlights of this edition:
Modeling human pain with organoids that recreate sensory pathways.
Lab-grown liver organoids now capture full metabolic zonation across all three zones.
A new protocol keeps adult hepatocytes both functional and growing long-term.
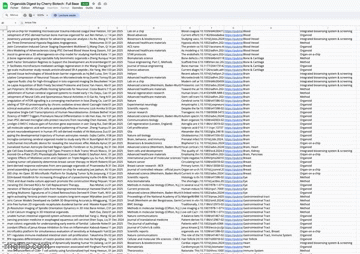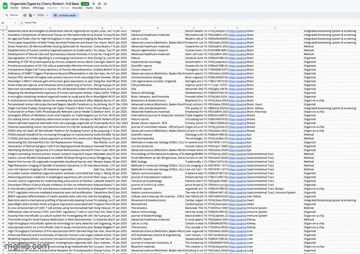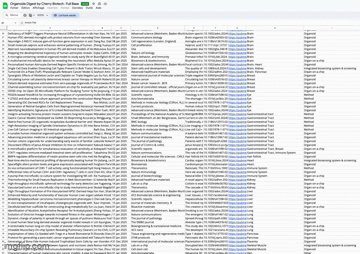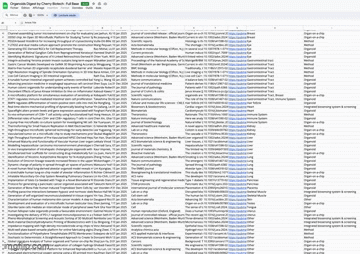
Full database of latest scientific paper on organoids with “Organoids Digest Extended”
More details below 👇
Welcome to Organoids Digest, your bi-weekly update on organoids, organ-on-a-chip, and 3D cell culture.
Within 2 weeks Nature published 3 papers on organoids, which is very exceptional. Is it because of the recent FDA announcement about phasing out animal testing for monoclonal antibodies and other drugs? We can’t tell, but anyway it’s a great signal for the development of new methodologies alternatives.
We wish you an enjoyable read ❤️
Article: Human assembloid model of the ascending neural sensory pathway
Ji-il Kim et al | April 09, 2025 | Nature
Can we finally model human pain?
Imagine being unable to feel pain even when severely injured, or, worse, experiencing relentless pain with no clear cause.
For decades, pain research has leaned on animal models or isolated neurons in dishes. But these fall short, they miss the human-specific biology and complexity of our neural circuitry.
Now, in a breakthrough from Stanford, scientists have constructed the first human-derived model of the ascending somatosensory pathway, the key route that carries pain and touch signals from body to brain.
What’s inside the model:
Built from four iPSC-derived organoids representing
Peripheral sensory neurons
Dorsal spinal cord
Thalamus
Cerebral cortex
Functions as a single, interconnected sensory network with synchronized neural activity across regions
What they demonstrate:
Incorporates SCN9A mutations (NaV1.7 channel), linked to congenital insensitivity or chronic pain
Reveals how these mutations disrupt full-circuit synchrony
Uncovers species-specific drug responses (TNP-ATP is a potent P2X3 receptor agonist in mice, not in human)
So what? We’re not quite there yet. Pain is deeply personal, shaped by more than neurons. But traditional models haven’t bridged that human gap. This assembloid brings us closer. By replicating essential, human-specific pain pathways in vitro, it could influence how we study, and treat, chronic pain.
Article: Multi-zonal liver organoids from human pluripotent stem cells
Hasan Al Reza et al | April 16 2025 | Nature
Zoned for function: rebuilding the liver’s invisible architecture
When examining most organs, it’s easy to see how structure reflects function. The liver? Not so much.
To the naked eye, it looks uniform. But each tiny liver unit, called a lobule, is finely zoned from the nutrient-rich portal triad (zone 1), through a middle band (zone 2), to the oxygen-poor central vein (zone 3).
Each zone does something different:
Zone 1 handles energy production, cholesterol synthesis, and kicks off ammonia detox
Zone 2 balances metabolism and leads regeneration after injury
Zone 3 specializes in drug breakdown and final detox steps
The breakthrough: a self-organizing multi-zonal liver organoid (mZ-HLO)
Scientists have now built an in vitro liver model that recreates all three zones; something no previous system had achieved:
Zone 1 cells made with vitamin C cues
Zone 3 cells shaped by bilirubin
In between, zone 2 forms naturally, filling the gap with regeneration-ready, antioxidant-rich cells
Single-cell analysis confirmed that these mini-livers match real human liver tissue better than eight other models.
Why it matters:
More accurate drug testing: Different zones react to different drugs, this model can catch those effects
Physiological ammonia handling and detox
Zone-specific injury and regeneration as shown by acetaminophen-mediated insult
Improved therapy potential: Transplanted organoids reduced liver stress and improved survival in rats
Article: Generation of human adult hepatocyte organoids with metabolic functions
Ryo Igarashi et al | April 16, 2025 | Nature
The hepatocyte dilemma solved : Growth without losing function
The human liver is unique, it can regenerate completely after injury.
But there’s a catch: once adult hepatocytes are isolated and cultured in vitro, they face a stark trade-off.
They can either:
Proliferate over time, but lose their liver-specific functions
orRemain mature and functional, but at the cost of their expansion capacity
This limitation has long stalled progress in liver research and drug development.
A new study offers a solution.
By using a precisely tuned mix of growth factors, most notably the cytokine oncostatin M, researchers have grown 3D liver organoids from single adult human hepatocytes that:
Expand over a million-fold in six months
Maintain their hepatocyte identity, without drifting into ductal-like cells
Upon differentiation in a hormone-rich medium, these organoids regained full liver functionality:
Albumin and clotting factor secretion
Bile acid, triglyceride, and cholesterol synthesis
Zonation-specific metabolism: gluconeogenesis, urea cycle, β-oxidation, CYP activity
Clinically relevant responses to acetaminophen toxicity, reversible by N-acetyl-cysteine
Why it matters: This protocol resolves the long-standing dilemma between growth and function. The result is a scalable, human-relevant liver model, enabling:
Accurate drug toxicity screening
Advanced liver disease modeling
Better tools for regenerative medicine
Organoids Digest Full Database
📄 Want even more recent and sorted resources? Check out the Organoids Digest Extended! It’s your go-to source for the latest scientific publications in organoids, organ-on-a-chip, and cutting-edge 3D models sorted by tissue and systems. Explore it by clicking below 👇
❤️ Thank you for reading this edition of Organoids Digest. If you enjoyed it, let us know by clicking the heart button below
🌐 Visit our site to learn more about 3D cell culture and organ-model know-how: cherrybiotech.com